Salts Are Always Double Covalent Compounds
Complex compounds with certain ligands have the ability to aid in the transformation of molecules in a catalytic or a stoichiometric manner. Isotopes of an.

Types Of Chemical Reactions Predicting Products Chemistry Etsy In 2021 Chemistry Worksheets Chemistry Chemistry Notes
The definition of a binary molecular compound is any compound that is made of two elements that are bonding together by a covalent bond.

. Double Salts and Coordination Complex Double Salts. Binary Covalent Compounds or Binary Molecular Compounds. Or it may be heteronuclear a chemical compound composed of more than one element as with water two hydrogen atoms and one oxygen.
One of the bonds is a double bond and thus the valency of carbon is satisfied. The number of electrons contributed by each atom for sharing. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds.
Covalent bonds are formed by the sharing of electrons between two atoms so that both can achieve a completely filled outermost shell. Naming salts and basic ionic compounds follows standard ionic nomenclature rules. In the kinetic theory of gases the term molecule is.
A soap molecule has a tadpole shaped structure. The shapes of stearic and oleic acids are displayed in the models below. Carbon always forms covalent bonds.
Covalent bonds are directional where the atoms that are bonded showcase specific orientations relative to one another. 234 is a time series of drops of food coloring diffusing in water. Double salts are completely ionizable in aqueous solutions and each ion in the solution gives the corresponding confirmatory test.
Covalent compounds like sugar and food coloring can dissolve and diffuse but they do not dissociate. The trans-double bond isomer of oleic acid known as elaidic acid has a linear shape and a melting point of 45 ºC 32 ºC higher than its cis isomer. Ionic compounds are often found as salts solid substances that usually separate and exist as individual ions in water.
You may examine models of these compounds by clicking on the desired model picture. By convention the first letter of an elements symbol is always capitalized while the second letter if present is lowercase. In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
All other ionic compounds without these ions are known as salts. A molecule is a group of two or more atoms held together by chemical bonds. Compounds with covalent bonds usually have lower enthalpies of vaporization and fusion.
Most compounds having covalent bonds exhibit relatively low melting points and boiling points. Carbon always forms a covalent bond. A molecule may be homonuclear that is it consists of atoms of one chemical element as with two atoms in the oxygen molecule O 2.
Thus the symbol for hydrogen is H the symbol for sodium is Na and the symbol for nickel is Ni. These two result from either the sharing of outer shell electrons or the transfer of the electrons from one atom to another. Most symbols come from the English name of the element although some symbols come from an elements Latin name.
The molecules of soap are sodium or potassium salts of long chain carboxylic acids. Depending on context the term may or may not include ions which satisfy this criterion. Carbon also forms compounds containing double and triple bonds between carbon atoms.
All other ionic compounds without these ions are known as salts. A chemical bond formed between two atoms by sharing of valence electrons between two atoms so that each atom acquires the stable electronic configuration of the nearest noble gas. In naming acids from binary compounds the prefix hydro- is used to represent the cation H and the suffix -ic acid is used to indicate that it is an acidic form.
Carbon forms covalent bonds with itself and other elements such as hydrogen oxygen sulphur nitrogen and chlorine. In naming acids from binary compounds the prefix hydro- is used to represent the cation H and the suffix -ic acid is used to indicate that it is an acidic form. Compounds and molecules are formed when atoms form either ionic or covalent bonds.
Naming salts and basic ionic compounds follows standard ionic nomenclature rules. Atomic no of carbon is 6. Without stirring the food coloring will mix into the water through only the movement of the water and food coloring molecules.

How Can You Differentiate Among Reactions In Aqueous Solutions Ppt Download
Why Is Acetone Soluble In Oil And Water Quora

Water Salt And The Hydrologic Cycle Snowflakes Science Frozen Party Activities Snowflake Photos

Acidic Basic And Neutral Salts Ionic Compounds Youtube
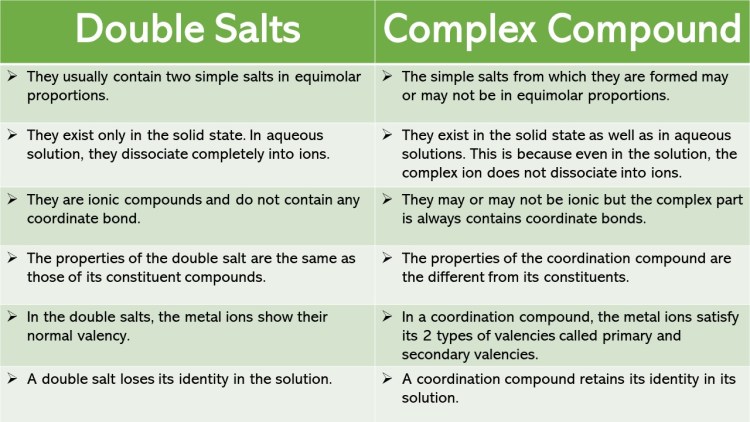
Coordination Compounds And Double Salts

Chemical Reactions Posters Part Ii Chemistry Classroom Chemistry Education Chemistry Lessons

Lecture 12 Chemical Bonding Lectures Notes Lecture Bond

Assault Battery And Grievous Bodily Harm Cases Chemistry Jokes Science Puns Nerd Jokes

Without Ionic Bonds There Would Be No Table Salt Ionic Bonding Ionic Ionic Compound

Organic Chemistry Science Educational School Posters Organic Chemistry Chemistry Classroom Teaching Chemistry

Pin By Sanghita Dey On Cbse Class 10 Covalent Bonding Sample Paper Molecular

Click To Download Redox Rules Posters For Vce Chemistry Teaching Chemistry High School Science Ap Chemistry

Pin By L Loyd On Chemistry Graphic Organizers Chemistry Education Teaching Chemistry How To Learn Chemistry

Calculate Heat Precipitation Example Problems Solutions 1 Precipitation What Is Heat Problem And Solution

Ionic Vs Covalent Bonds Ionic Vs Covalent Bonds Covalent Bonding Covalent Bonds

Click To Download Redox Rules Posters For Vce Chemistry Teaching Chemistry High School Science Ap Chemistry

Construction Of The Lewis Electron Dot Structure Of Cn2h2 Canonical Form Electrons Lewis


Comments
Post a Comment